| Catalog# | Product Name | Size | Price | Qty | Inquiry |
|---|---|---|---|---|---|
| THP-0138 | Ocriplasmin, Recombinant truncated human plasmin | 50ug | $3,998.00 |
|
Add to Cart Order |
| THP-0184 | Becaplermin, Recombinant Human PDGFB | 1 vial | $3,998.00 |
|
Add to Cart Order |
Alpha-2-Macroglobulin (A2M), a multifunctional plasma glycoprotein, plays a critical role in protease inhibition, inflammation and tissue remodeling. Found in plasma and various tissues, A2M plays a central role in immune modulation and extracellular matrix stabilization. Its dysregulation has been implicated in several pathologies, including Alzheimer's disease, osteoarthritis, cancer and chronic inflammation. Advances in biotechnology have fostered the development of therapeutic proteins targeting A2M-regulated pathways, providing novel avenues for clinical intervention.
NCBI Gene ID: 2
UniProtKB ID: P01023
Alpha-2-macroglobulin (A2M) is a large (~720 kDa) plasma glycoprotein composed of four identical subunits arranged in a tetrameric structure. It belongs to the macroglobulin protein family and is highly conserved across species. Each subunit contains:
A2M is predominantly synthesized in the liver but is also produced by macrophages, fibroblasts, and other cells under inflammatory conditions.
A2M serves as a broad-spectrum protease inhibitor, regulating proteolytic activity in plasma and tissues. Its major functions include:
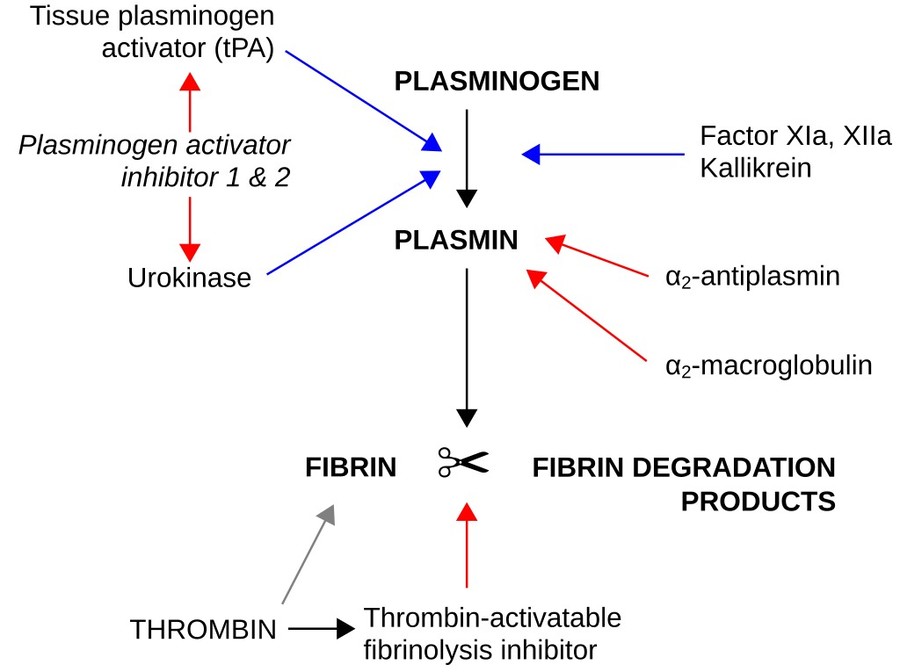 Figure 1. Fibrinolysis (simplified). Blue arrows
denote stimulation, and red arrows inhibition. α2-Macroglobulin functions as an inhibitor of fibrinolysis by inhibiting plasmin and kallikrein. And it
functions as an inhibitor of coagulation by inhibiting thrombin.
Figure 1. Fibrinolysis (simplified). Blue arrows
denote stimulation, and red arrows inhibition. α2-Macroglobulin functions as an inhibitor of fibrinolysis by inhibiting plasmin and kallikrein. And it
functions as an inhibitor of coagulation by inhibiting thrombin.
A2M exerts its protective role by, including serine, cysteine and metalloproteinases, via a bait and trap mechanism. It sequesters proteases, rendering them inactive and facilitating their clearance via receptor-mediated endocytosis. Dysregulated protease activity in the absence of adequate A2M function contributes to pathological tissue damage, inflammation and impaired wound healing.
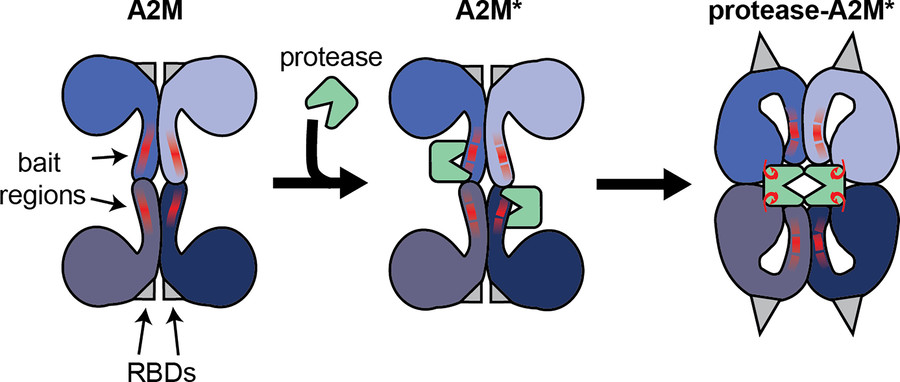 Figure 2. Illustration of the interaction between the
A2M tetramer and active endopeptidases. Functional A2M is formed by a non-covalent interaction between two covalently linked dimers. Each monomer contains a
protease bait region (red lines) and a buried receptor binding domain (RBDs, gray triangles). Active proteases can cleave the bait region, resulting in A2M
activation (A2M*) and conformational rearrangement. This process results in physical trapping of the protease and the exposure of a reactive thioester bond
which may result in the formation of a covalent bond between A2M and protease lysine residues (red hooks). Simultaneously, the receptor binding domains are
exposed to the protein surface, thereby enabling A2M* to bind its cell-surface receptors. (Vandooren and Itoh, 2021)
Figure 2. Illustration of the interaction between the
A2M tetramer and active endopeptidases. Functional A2M is formed by a non-covalent interaction between two covalently linked dimers. Each monomer contains a
protease bait region (red lines) and a buried receptor binding domain (RBDs, gray triangles). Active proteases can cleave the bait region, resulting in A2M
activation (A2M*) and conformational rearrangement. This process results in physical trapping of the protease and the exposure of a reactive thioester bond
which may result in the formation of a covalent bond between A2M and protease lysine residues (red hooks). Simultaneously, the receptor binding domains are
exposed to the protein surface, thereby enabling A2M* to bind its cell-surface receptors. (Vandooren and Itoh, 2021)
Harnessing A2M as a therapeutic target requires innovative approaches to modulate its activity in a disease-specific context. Current therapeutic proteins such as ocriplasmin and becaplermin demonstrate the feasibility of targeting A2M-mediated protease pathways and inflammation. However, additional recombinant proteins and small molecules designed to enhance or mimic A2M function remain to be explored.
Recent studies suggest that A2M-based therapies could treat neurodegenerative diseases such as Alzheimer's disease by reducing amyloid-beta aggregation. Similarly, in oncology, A2M may inhibit tumor progression by neutralizing tumor-associated proteases. Translating these findings into clinical applications requires further investigation of A2M's structure, interaction networks, and regulatory mechanisms.
Creative BioMart specializes in providing therapeutic proteins, including ocriplasmin and becaplermin, which target alpha-2 macroglobulin. Contact us with any questions or inquiries.
References
For more information on how our products could help advance your project, please contact us.
ENTER YOUR EMAIL HERE TO SUBSCRIBE.
Copyright © 2025 Creative BioMart. All Rights Reserved.