Angiopoietin 1 (Ang1), encoded by the gene ANGPT1, is a ligand of the Tie2 receptor, a critical regulator of vascular stability, endothelial cell
survival and angiogenesis. Dysregulation of Angiopoietin 1-Tie2 signaling is associated with several pathological conditions, including cancer,
inflammation, and vascular leakage syndromes. Vascular homeostasis is maintained by a dynamic balance of pro- and anti-angiogenic signals in which the
angiopoietin-Tie2 axis plays a central role. Ang1 activates the Tie2 receptor, promoting vascular maturation, stability and anti-inflammatory effects.
Conversely, its antagonist, angiopoietin 2 (encoded by the gene ANGPT2), disrupts these effects, leading to vascular
destabilization and pathological angiogenesis. Given its central role in vascular function, Ang1 has become a compelling target for therapeutic intervention
in diseases involving aberrant angiogenesis or vascular permeability.
NCBI Gene ID: 284
UniProtKB ID: Q15389
The Angiopoietin-Tie2 Axis: A Key Regulator of Vascular Stability
Key Components of the Ang-Tie2 Axis
Tie2 Receptor
- A tyrosine kinase receptor primarily expressed on endothelial cells.
- Activation of Tie2 enhances vascular quiescence, anti-inflammatory effects, and endothelial survival.
Angiopoietins
- Angiopoietin-1: A Tie2 agonist that stabilizes blood vessels, strengthens endothelial junctions, and prevents vascular leakage.
-
Angiopoietin-2: A context-dependent antagonist that competes with Ang1 for Tie2 binding. At baseline, it supports vascular remodeling, but in disease conditions, it
destabilizes vessels, increases permeability, and enhances inflammation.
- Angiopoietin-4: Structurally similar to Ang1 but with less-defined functions in humans.
- Angiopoietin-like Proteins: Functionally related proteins that regulate lipid metabolism and angiogenesis.
Mechanisms of Ang-Tie2 Signaling
Ang1 Binding to Tie2 → Pro-Stabilization Pathway
- Strengthens endothelial cell–cell junctions.
- Reduces vascular permeability and inflammation.
- Suppresses pro-inflammatory signals, protecting against vascular leakage syndromes.
Ang2 Binding to Tie2 → Destabilization Pathway
- In quiescent vessels, Ang2 acts as a weak Tie2 agonist, enabling vascular plasticity.
-
Under inflammatory or hypoxic conditions, Ang2 inhibits Tie2 activity, leading to endothelial destabilization, increased permeability, and inflammatory
cell infiltration.
- High Ang2/Ang1 ratios correlate with vascular dysfunction in diseases like sepsis, cancer, and chronic inflammation.
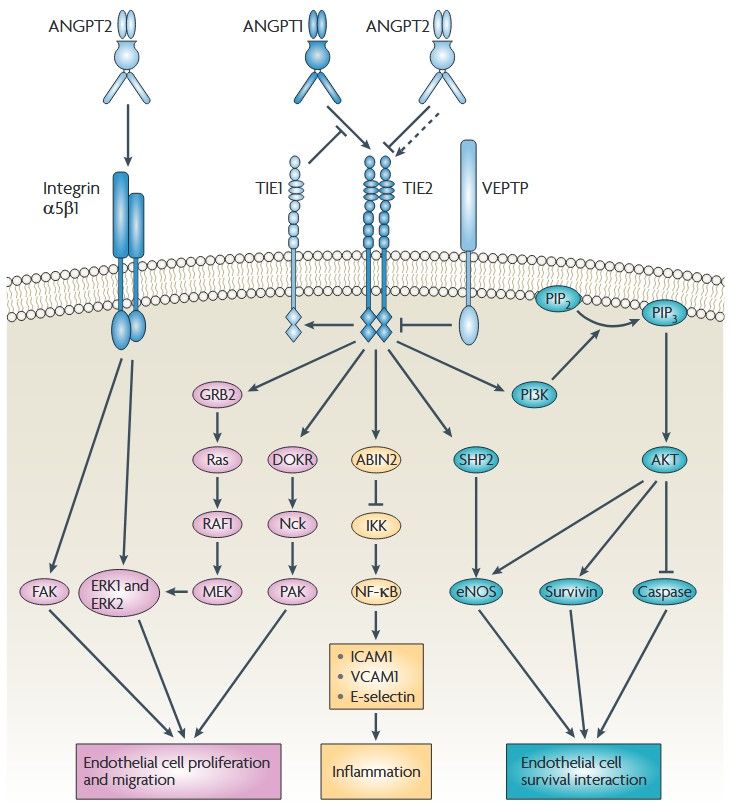 Figure 1. The ANGPT-Tie signaling pathways.
Angiopoietin 1 (ANGPT1) multimers bind to Tie2 on endothelial cells and Tie2 dimerizes or multimerizes and is auto phosphorylated. Activated Tie2 binds and
activates several other effectors, including downstream of tyrosine kinase-related (DOKR; also known as DOK2), endothelial nitric oxide synthase (eNOS), SH2
domain-containing phosphatase (SHP2), growth factor receptor-bound protein 2 (GRB2) and the p85 subunit of PI3K19–21 . SHP2 and PI3K mediate the activation
of eNOS and so increase NO production. Conversely, ANGPT2 normally functions as an ANGPT1 antagonist and mediates increases in vascular permeability and
primes the vasculature for angiogenesis. (Huang et al., 2010)
Figure 1. The ANGPT-Tie signaling pathways.
Angiopoietin 1 (ANGPT1) multimers bind to Tie2 on endothelial cells and Tie2 dimerizes or multimerizes and is auto phosphorylated. Activated Tie2 binds and
activates several other effectors, including downstream of tyrosine kinase-related (DOKR; also known as DOK2), endothelial nitric oxide synthase (eNOS), SH2
domain-containing phosphatase (SHP2), growth factor receptor-bound protein 2 (GRB2) and the p85 subunit of PI3K19–21 . SHP2 and PI3K mediate the activation
of eNOS and so increase NO production. Conversely, ANGPT2 normally functions as an ANGPT1 antagonist and mediates increases in vascular permeability and
primes the vasculature for angiogenesis. (Huang et al., 2010)
Biological Role of Ang1
Ang1 plays dual and context-dependent roles in vascular homeostasis and angiogenesis, primarily through its interaction with the Tie2 receptor on
endothelial cells. Its functions vary between stable vessels and sprouting angiogenesis, ensuring both vascular integrity and controlled vessel formation.
In quiescent, mature vasculature, Ang1 is essential for vascular stabilization and maintenance of endothelial integrity. Through Tie2 activation, Ang1:
- Strengthens endothelial cell-cell junctions, reducing vascular permeability.
- Suppresses inflammatory signaling, preventing leukocyte infiltration and endothelial activation.
- Promotes pericyte recruitment and vessel maturation, reinforcing vascular stability.
- Enhances endothelial survival and quiescence, reducing apoptosis and unnecessary vessel remodeling.
During angiogenesis, Ang1 coordinates vessel sprouting while preventing excessive permeability and instability:
- Modulates endothelial cell migration and proliferation, enabling controlled sprouting.
- Supports pericyte attachment to nascent vessels, ensuring proper vessel stabilization.
- Balances VEGF-driven angiogenesis, preventing uncontrolled vessel growth and leakiness.
- Maintains Tie2 activation in sprouting endothelial cells, preserving vascular integrity during dynamic remodeling.
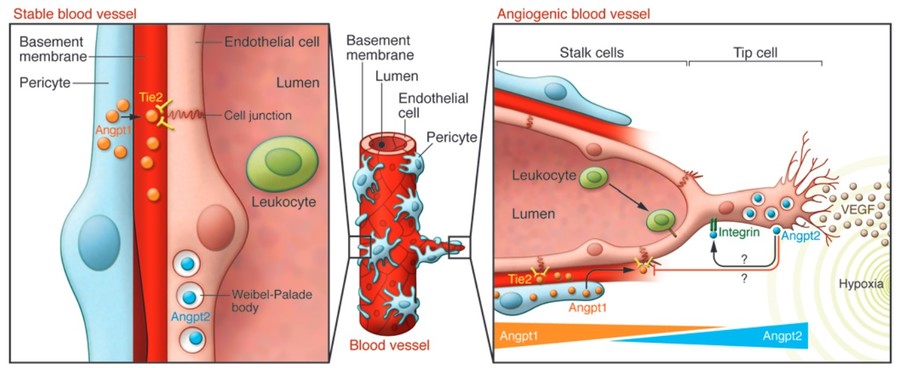 Figure 2. The ANGPT-Tie system in stable vessels
and sprouting angiogenesis. (Saharinen and Alitalo, 2011)
Figure 2. The ANGPT-Tie system in stable vessels
and sprouting angiogenesis. (Saharinen and Alitalo, 2011)
Ang1 Dysregulation in Disease
Altered Ang1 signaling is implicated in multiple pathological conditions:
-
Cancer: Tumors exploit angiogenesis to ensure adequate blood supply for growth and metastasis. Increased Ang2 levels in the tumor microenvironment often
overwhelm Ang1-mediated stabilization, contributing to disorganized and leaky vasculature.
-
Vascular Leakage Syndromes: Conditions such as sepsis and acute respiratory distress syndrome (ARDS) involve endothelial barrier
dysfunction, where insufficient Ang1 activity exacerbates vascular leakage.
-
Chronic Inflammation: Dysregulated angiogenesis and vascular instability in chronic inflammatory diseases are partly due to imbalanced
Ang1 and Ang2 signaling.
Therapeutic Targeting of Ang1
Given its role in vascular homeostasis, Ang1 has been targeted in therapeutic development, either to restore its function or to modulate its effects in
disease contexts.
-
Trebananib (AMG 386): Trebananib is a peptide-Fc fusion protein that inhibits the interaction of Ang1
and Ang2 with the Tie2 receptor. By preventing both ANGPTs from binding, trebananib disrupts angiogenesis, specifically in tumor vasculature. It has
demonstrated efficacy in clinical trials for cancers such as ovarian carcinoma, where its anti-angiogenic properties inhibit tumor growth and
progression. Unlike traditional VEGF inhibitors, trebananib targets a distinct pathway, potentially reducing resistance and offering combinatory
benefits with other therapies.
-
Ang1 Agonists: Therapeutic strategies are also exploring the direct activation of Ang1-Tie2 signaling to stabilize vasculature in
conditions like sepsis and diabetic retinopathy. Recombinant Ang1 mimetics or Tie2 agonists aim to restore vascular integrity and reduce pathological
leakage.
-
Gene Therapy Approaches: Gene delivery systems for Ang1 are being developed to enhance its expression in ischemic tissues or
inflammatory environments, promoting vessel stabilization and repair.
Challenges and Future Directions
While targeting Ang1 offers significant therapeutic promise, several challenges remain:
-
Complex Interplay with Ang2: The dual role of Ang1 and Ang2 in Tie2 signaling complicates therapeutic design, as both ligands are
tightly interrelated.
-
Off-Target Effects: Ang1's systemic effects on the vasculature may result in unintended consequences, such as impaired wound healing or
excessive vessel stabilization.
-
Tumor Resistance Mechanisms: Tumors may compensate for Ang1-Tie2 pathway inhibition by upregulating alternative angiogenic pathways,
necessitating combination therapies.
Future research should focus on developing more selective Ang1 modulators and exploring their use in combination with
VEGF inhibitors or immunotherapies. Advanced delivery methods, such as nanoparticles or
tissue-specific targeting, may also enhance therapeutic efficacy while minimizing systemic side effects.
At Creative BioMart, we provide cutting-edge therapeutic proteins like trebananib targeting angiopoietin 1.
Contact us for more details!
References
-
Brindle NPJ, Saharinen P, Alitalo K. Signaling and functions of angiopoietin-1 in vascular protection. Circulation Research.
2006;98(8):1014-1023. doi:10.1161/01.RES.0000218275.54089.12
-
Huang H, Bhat A, Woodnutt G, Lappe R. Targeting the ANGPT–TIE2 pathway in malignancy. Nat Rev Cancer. 2010;10(8):575-585. doi:10.1038/nrc2894
- Saharinen P, Alitalo K. The yin, the yang, and the Angiopoietin-1. J Clin Invest. 2011;121(6):2157-2159. doi:10.1172/JCI58196
 Figure 1. The ANGPT-Tie signaling pathways.
Angiopoietin 1 (ANGPT1) multimers bind to Tie2 on endothelial cells and Tie2 dimerizes or multimerizes and is auto phosphorylated. Activated Tie2 binds and
activates several other effectors, including downstream of tyrosine kinase-related (DOKR; also known as DOK2), endothelial nitric oxide synthase (eNOS), SH2
domain-containing phosphatase (SHP2), growth factor receptor-bound protein 2 (GRB2) and the p85 subunit of PI3K19–21 . SHP2 and PI3K mediate the activation
of eNOS and so increase NO production. Conversely, ANGPT2 normally functions as an ANGPT1 antagonist and mediates increases in vascular permeability and
primes the vasculature for angiogenesis. (Huang et al., 2010)
Figure 1. The ANGPT-Tie signaling pathways.
Angiopoietin 1 (ANGPT1) multimers bind to Tie2 on endothelial cells and Tie2 dimerizes or multimerizes and is auto phosphorylated. Activated Tie2 binds and
activates several other effectors, including downstream of tyrosine kinase-related (DOKR; also known as DOK2), endothelial nitric oxide synthase (eNOS), SH2
domain-containing phosphatase (SHP2), growth factor receptor-bound protein 2 (GRB2) and the p85 subunit of PI3K19–21 . SHP2 and PI3K mediate the activation
of eNOS and so increase NO production. Conversely, ANGPT2 normally functions as an ANGPT1 antagonist and mediates increases in vascular permeability and
primes the vasculature for angiogenesis. (Huang et al., 2010)
 Figure 2. The ANGPT-Tie system in stable vessels
and sprouting angiogenesis. (Saharinen and Alitalo, 2011)
Figure 2. The ANGPT-Tie system in stable vessels
and sprouting angiogenesis. (Saharinen and Alitalo, 2011)