Monoclonal antibodies (mAbs) are a cornerstone of modern therapeutic proteins, revolutionizing the treatment of diseases ranging from cancer to autoimmune disorders. These highly specific proteins are designed to target specific antigens, providing precision medicine with fewer off-target effects than traditional small molecule drugs.
Explore Creative BioMart's extensive monoclonal antibody portfolio and discover their background, key benefits, and unique product advantages.
What Are Monoclonal Antibodies?
Monoclonal antibodies are laboratory-produced molecules designed to mimic the immune system's ability to fight harmful pathogens. They are derived from a single B-cell clone, ensuring uniformity and specificity in targeting a single epitope on an antigen. This specificity makes mAbs powerful tools for the diagnosis and treatment of disease.
Basic Structure
Monoclonal antibodies have a well-defined Y-shaped structure composed of four polypeptide chains:
- Two heavy chains (approximately 50 kDa each)
- Two light chains (approximately 25 kDa each)
These chains are connected by disulfide bonds, forming a stable and functional antibody molecule. The mAb structure consists of two key regions that determine its function:
Fab Region (Fragment Antigen-Binding)
- Located at the tips of the "Y" shape, this region contains variable domains that enable antigen recognition.
- The variable regions consist of hypervariable loops known as complementarity-determining regions (CDRs), which provide specificity to the target antigen.
- Fab fragments can be used independently as therapeutic agents, particularly in applications where minimal immune system involvement is required.
Fc Region (Fragment Crystallizable)
- The stem of the antibody, which interacts with immune cells and effector molecules.
- This region determines the antibody's isotype (e.g., IgG, IgA, IgE) and mediates immune system interactions, such as binding to Fc receptors on immune cells and activation of complement pathways.
- Modifications in the Fc region can enhance antibody half-life, reduce immunogenicity, or improve therapeutic efficacy.
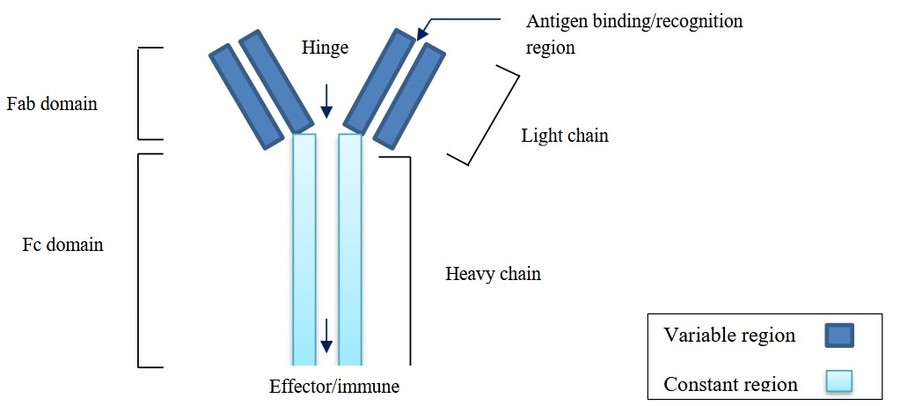 Figure 1. Structure of monoclonal antibody and its components. (Dabhadkar et al., 2022)
Figure 1. Structure of monoclonal antibody and its components. (Dabhadkar et al., 2022)
Mechanisms of Action
Neutralization
- mAbs bind to pathogens, toxins, or receptor-ligand interactions, preventing them from exerting harmful effects.
- Example: Palivizumab, a monoclonal antibody targeting the respiratory syncytial virus (RSV), prevents viral entry into host cells.
Opsonization and Phagocytosis
- By coating pathogens or abnormal cells, mAbs mark them for recognition and destruction by phagocytic immune cells (e.g., macrophages, neutrophils).
- Example: Rituximab, which targets CD20 on B cells, facilitates the clearance of malignant and autoimmune B cells.
Antibody-Dependent Cellular Cytotoxicity (ADCC)
- mAbs engage Fc receptors on immune cells such as natural killer (NK) cells, triggering the release of cytotoxic granules that induce apoptosis in target cells.
- Example: Trastuzumab, used for HER2-positive breast cancer, enhances ADCC-mediated tumor cell destruction.
Complement-Dependent Cytotoxicity (CDC)
- The Fc region of mAbs can activate the complement cascade, leading to the formation of the membrane attack complex (MAC) that lyses target cells.
- Example: Rituximab mediates CDC to eliminate B-cell malignancies.
Checkpoint Inhibition and Immune Modulation
- Some mAbs block inhibitory receptors on immune cells, restoring immune system activity against cancer cells.
- Example: Pembrolizumab, an anti-PD-1 monoclonal antibody, enhances T-cell-mediated tumor destruction.
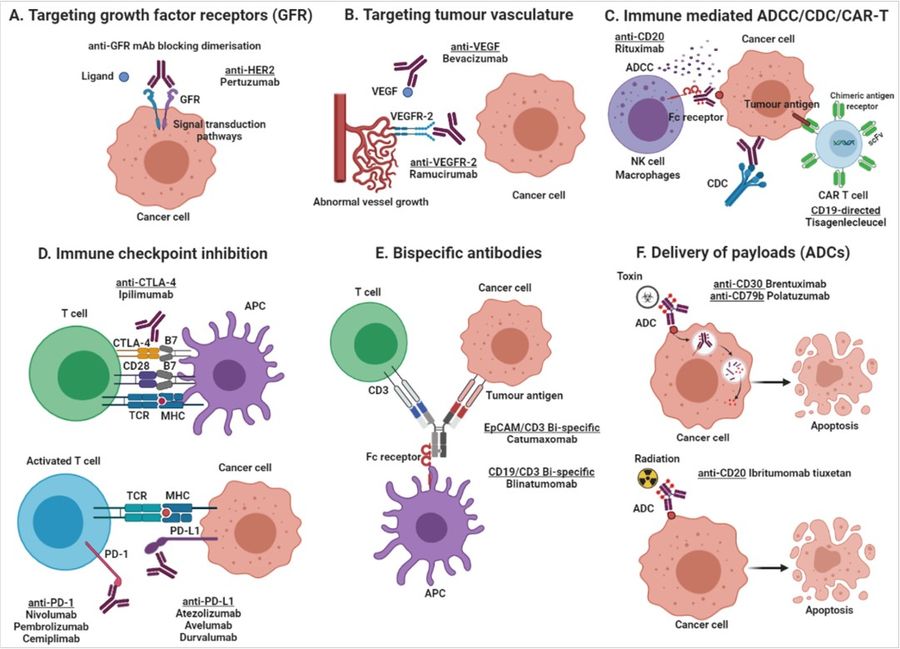 Figure 2. Mechanisms of action of monoclonal antibody-based products. (A) Targeting growth factor receptors, blocking the binding of an activating ligand and inhibiting receptor homo- and heterodimerization; (B) Targeting of tumor vasculature receptor or its ligands inhibiting angiogenesis; (C) Induction of apoptosis by recruitment of immune effector cells (ADCC) or activation of the complement cascade (CDC), and the use of antibody-based molecules to engineer T lymphocytes (CAR T cells); (D) Immune checkpoint inhibition by blockade of the PD-1/PD-L1 axis or CTLA-4 inhibitory receptors, increasing cytotoxic T cell activity; (E) Simultaneous targeting of two antigens, one on tumor cells and one on effector T cells, by using bispecific antibodies (BITE, bispecific T-cell enhancing); and (F) Delivery of payloads such as toxins and radioisotopes to tumor cells. (Arias-Pinilla and Modjtahedi, 2021)
Figure 2. Mechanisms of action of monoclonal antibody-based products. (A) Targeting growth factor receptors, blocking the binding of an activating ligand and inhibiting receptor homo- and heterodimerization; (B) Targeting of tumor vasculature receptor or its ligands inhibiting angiogenesis; (C) Induction of apoptosis by recruitment of immune effector cells (ADCC) or activation of the complement cascade (CDC), and the use of antibody-based molecules to engineer T lymphocytes (CAR T cells); (D) Immune checkpoint inhibition by blockade of the PD-1/PD-L1 axis or CTLA-4 inhibitory receptors, increasing cytotoxic T cell activity; (E) Simultaneous targeting of two antigens, one on tumor cells and one on effector T cells, by using bispecific antibodies (BITE, bispecific T-cell enhancing); and (F) Delivery of payloads such as toxins and radioisotopes to tumor cells. (Arias-Pinilla and Modjtahedi, 2021)
Types of Monoclonal Antibodies
Murine mAbs
- Derived from mice, these antibodies are fully murine in origin and can trigger immune responses in humans, leading to rapid clearance and reduced efficacy.
- Example: Muromonab-CD3 (OKT3) – used in organ transplant rejection but largely replaced by newer mAbs due to immunogenicity concerns.
Chimeric mAbs
- These antibodies contain murine variable regions fused with human constant regions, reducing immunogenicity while maintaining antigen specificity.
- Example: Rituximab, a chimeric anti-CD20 antibody, is widely used in B-cell lymphomas and autoimmune diseases.
Humanized mAbs
- These antibodies are predominantly human, with only the antigen-binding sites derived from murine sequences. They offer reduced immunogenicity while preserving antigen specificity.
- Example: Trastuzumab, a humanized anti-HER2 antibody, is a key treatment for HER2-positive breast cancer.
Fully Human mAbs
- Produced using advanced technologies such as transgenic mice (with human antibody genes) or phage display, these antibodies have minimal immunogenicity.
- Example: Adalimumab, a fully human anti-TNFα antibody, is widely used for autoimmune conditions like rheumatoid arthritis and Crohn's disease.
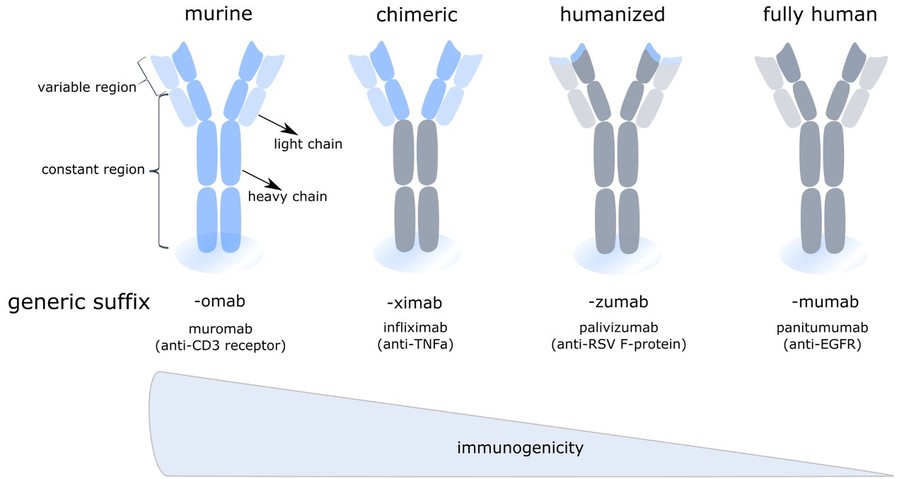 Figure 3. Overview of monoclonal antibody variants used in therapy. Next to classical fully murine (left) or human monoclonal antibodies (right), recombinant species are used in therapy (middle). These include chimeric mAbs, composed of human constant regions and murine variable regions, and humanized mAbs, where the hypervariable CDR-domains of the murine antibody are grafted on a human antibody. Clinically applied examples of each are given, including their targets between brackets (Van Hoecke and Roose, 2019)
Figure 3. Overview of monoclonal antibody variants used in therapy. Next to classical fully murine (left) or human monoclonal antibodies (right), recombinant species are used in therapy (middle). These include chimeric mAbs, composed of human constant regions and murine variable regions, and humanized mAbs, where the hypervariable CDR-domains of the murine antibody are grafted on a human antibody. Clinically applied examples of each are given, including their targets between brackets (Van Hoecke and Roose, 2019)
Development of Monoclonal Antibodies
Production Technologies
- Hybridoma Technology: Fusing B-cells with myeloma cells to produce immortalized antibody-secreting cells.
- Phage Display: Screening libraries of antibody fragments displayed on bacteriophages to identify high-affinity binders.
- Transgenic Mice: Engineered to produce human antibodies, bypassing the need for humanization.
- Recombinant DNA Technology: Enables the production of mAbs in mammalian cell cultures (e.g., CHO cells).
Challenges in Development
- Immunogenicity: Human anti-mouse antibodies (HAMAs) can limit efficacy.
- Manufacturing Complexity: High costs and scalability issues in cell culture systems.
- Regulatory Hurdles: Stringent requirements for safety and efficacy testing.
Applications of Monoclonal Antibodies
Oncology
mAbs have transformed cancer treatment by targeting tumor-specific antigens and modulating the immune response. Examples include:
- Trastuzumab: Targets HER2/neu in breast cancer.
- Rituximab: Binds CD20 on B-cells in non-Hodgkin lymphoma.
- Pembrolizumab: A checkpoint inhibitor targeting PD-1 in melanoma and other cancers.
Autoimmune Diseases
mAbs modulate the immune system to treat autoimmune disorders:
Infectious Diseases
- Palivizumab: Prevents respiratory syncytial virus (RSV) in high-risk infants.
- Bamlanivimab: An emergency-authorized mAb for COVID-19.
Other Applications
- Cardiovascular Diseases: Evolocumab lowers LDL cholesterol by targeting PCSK9.
- Neurology: Aducanumab targets amyloid-beta plaques in Alzheimer's disease.
At Creative BioMart, we are your trusted supplier of high-quality monoclonal antibodies, ensuring exceptional consistency, reliability, and performance. Our monoclonal antibody products are meticulously developed to deliver high lot-to-lot reproducibility, guaranteeing accurate and reproducible results for research, diagnostic, and therapeutic applications. Contact us today to learn more details.
References
- Arias-Pinilla GA, Modjtahedi H. Therapeutic application of monoclonal antibodies in pancreatic cancer: advances, challenges and future opportunities. Cancers. 2021;13(8):1781. doi:10.3390/cancers13081781
- Dabhadkar MM, Palekar SR, Pawar KN. A review on "monoclonal antibodies: effective treatment against COVID-19." Int J Community Med Public Health. 2022;10(1):525. doi:10.18203/2394-6040.ijcmph20223584
- Van Hoecke L, Roose K. How mRNA therapeutics are entering the monoclonal antibody field. J Transl Med. 2019;17(1):54. doi:10.1186/s12967-019-1804-8

 Figure 1. Structure of monoclonal antibody and its components. (Dabhadkar et al., 2022)
Figure 1. Structure of monoclonal antibody and its components. (Dabhadkar et al., 2022) Figure 2. Mechanisms of action of monoclonal antibody-based products. (A) Targeting growth factor receptors, blocking the binding of an activating ligand and inhibiting receptor homo- and heterodimerization; (B) Targeting of tumor vasculature receptor or its ligands inhibiting angiogenesis; (C) Induction of apoptosis by recruitment of immune effector cells (ADCC) or activation of the complement cascade (CDC), and the use of antibody-based molecules to engineer T lymphocytes (CAR T cells); (D) Immune checkpoint inhibition by blockade of the PD-1/PD-L1 axis or CTLA-4 inhibitory receptors, increasing cytotoxic T cell activity; (E) Simultaneous targeting of two antigens, one on tumor cells and one on effector T cells, by using bispecific antibodies (BITE, bispecific T-cell enhancing); and (F) Delivery of payloads such as toxins and radioisotopes to tumor cells. (Arias-Pinilla and Modjtahedi, 2021)
Figure 2. Mechanisms of action of monoclonal antibody-based products. (A) Targeting growth factor receptors, blocking the binding of an activating ligand and inhibiting receptor homo- and heterodimerization; (B) Targeting of tumor vasculature receptor or its ligands inhibiting angiogenesis; (C) Induction of apoptosis by recruitment of immune effector cells (ADCC) or activation of the complement cascade (CDC), and the use of antibody-based molecules to engineer T lymphocytes (CAR T cells); (D) Immune checkpoint inhibition by blockade of the PD-1/PD-L1 axis or CTLA-4 inhibitory receptors, increasing cytotoxic T cell activity; (E) Simultaneous targeting of two antigens, one on tumor cells and one on effector T cells, by using bispecific antibodies (BITE, bispecific T-cell enhancing); and (F) Delivery of payloads such as toxins and radioisotopes to tumor cells. (Arias-Pinilla and Modjtahedi, 2021) Figure 3. Overview of monoclonal antibody variants used in therapy. Next to classical fully murine (left) or human monoclonal antibodies (right), recombinant species are used in therapy (middle). These include chimeric mAbs, composed of human constant regions and murine variable regions, and humanized mAbs, where the hypervariable CDR-domains of the murine antibody are grafted on a human antibody. Clinically applied examples of each are given, including their targets between brackets (Van Hoecke and Roose, 2019)
Figure 3. Overview of monoclonal antibody variants used in therapy. Next to classical fully murine (left) or human monoclonal antibodies (right), recombinant species are used in therapy (middle). These include chimeric mAbs, composed of human constant regions and murine variable regions, and humanized mAbs, where the hypervariable CDR-domains of the murine antibody are grafted on a human antibody. Clinically applied examples of each are given, including their targets between brackets (Van Hoecke and Roose, 2019)