| Catalog# | Product Name | Size | Price | Qty | Inquiry |
|---|---|---|---|---|---|
| THP-0020 | Tenecteplase; Modified Tissue plasminogen activator (tPA) | 100ug | $798.00 |
|
Add to Cart Order |
| 500ug | $2,498.00 |
|
Add to Cart Order | ||
| 1mg | $3,998.00 |
|
Add to Cart Order | ||
| THP-0052 | Recombinant Human F8/Antihemophilic factor | 100IU | $698.00 |
|
Add to Cart Order |
| THP-0085 | Moroctocog alfa (BDDrFVIII) | 100IU | $998.00 |
|
Add to Cart Order |
| 1,000IU | $3,998.00 |
|
Add to Cart Order | ||
| THP-0216 | Lonoctocog alfa, Recombinant Factor VIII | 1 vial | $3,998.00 |
|
Add to Cart Order |
Calnexin (encoded by the gene CANX, abbreviated as CNX) is an essential molecular chaperone involved in glycoprotein quality control in the endoplasmic reticulum (ER). It plays a critical role in maintaining cellular homeostasis and has been implicated in several diseases, including cancer, neurodegenerative disorders and viral infections. Given its functional importance in protein folding and cellular stress responses, calnexin has emerged as a potential drug target.
NCBI Gene ID: 821
UniProtKB ID: P27824
Calnexin is a 90 kDa protein characterized by an ER luminal domain, a single transmembrane segment and a cytosolic tail. It is part of the calnexin/calreticulin cycle that assists in the proper folding of nascent glycoproteins. Calnexin specifically recognizes monoglucosylated N-glycans on newly synthesized proteins and retains them in the ER until they reach their correct conformation. Interaction with the protein disulfide isomerase ERp57 further enhances its role in disulfide bond formation and quality control. This chaperone function is critical for cellular integrity and its dysfunction is implicated in several pathological conditions.
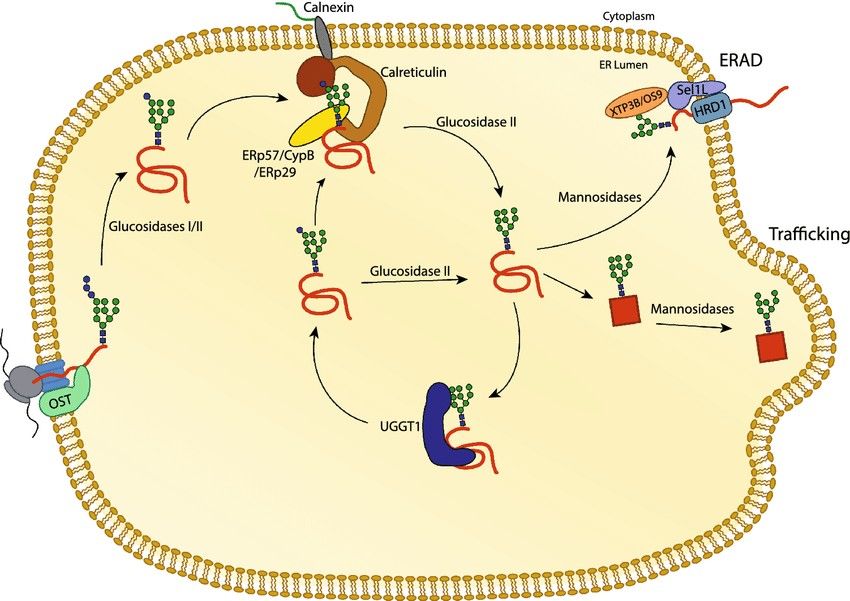 Figure 1. The calnexin/calreticulin substrate
binding cycle. Proteins targeted to the ER receive N-linked glycans that are transferred by the OST complex to acceptor sites. The first two glucoses are
trimmed by glucosidases I and II, leaving a monoglucosylated glycan. In this state, the glycan is a substrate for calnexin and calreticulin. Release from
calnexin/calreticulin and trimming of the final glucose by glucosidase II leaves the glycan in a non-glucosylated state. Productive folding and adoption of
a native state allows for trafficking of the glycoprotein from the ER. Glycoproteins that do not adopt a native fold can be recognized by the folding sensor
UDP-glucose: glycoprotein glucosyltransferase 1 (UGGT1). UGGT1 reglucosylates substrates, allowing for rebinding to calnexin/calreticulin or trimming by
glucosidase II. Glycoproteins that continue to non-productively fold can be removed from the calnexin/calreticulin cycle through trimming by mannosidases
and targeting to ER associated degradation (ERAD) machinery. (Adams et al., 2019)
Figure 1. The calnexin/calreticulin substrate
binding cycle. Proteins targeted to the ER receive N-linked glycans that are transferred by the OST complex to acceptor sites. The first two glucoses are
trimmed by glucosidases I and II, leaving a monoglucosylated glycan. In this state, the glycan is a substrate for calnexin and calreticulin. Release from
calnexin/calreticulin and trimming of the final glucose by glucosidase II leaves the glycan in a non-glucosylated state. Productive folding and adoption of
a native state allows for trafficking of the glycoprotein from the ER. Glycoproteins that do not adopt a native fold can be recognized by the folding sensor
UDP-glucose: glycoprotein glucosyltransferase 1 (UGGT1). UGGT1 reglucosylates substrates, allowing for rebinding to calnexin/calreticulin or trimming by
glucosidase II. Glycoproteins that continue to non-productively fold can be removed from the calnexin/calreticulin cycle through trimming by mannosidases
and targeting to ER associated degradation (ERAD) machinery. (Adams et al., 2019)
Creative BioMart is proud to supply comprehensive categories of therapeutic proteins, contact us with any questions or inquiries.
Reference
For more information on how our products could help advance your project, please contact us.
ENTER YOUR EMAIL HERE TO SUBSCRIBE.
Copyright © 2026 Creative BioMart. All Rights Reserved.