| Catalog# | Product Name | Size | Price | Qty | Inquiry |
|---|---|---|---|---|---|
| THP-0013 | Conestat alfa; Recombinant C1 esterase inhibitor | 1 vial | $3,998.00 |
|
Add to Cart Order |
| THP-0016 | Native Human C1-esterase inhibitor (hC1INH) | 1 vial | $3,998.00 |
|
Add to Cart Order |
| THP-0112 | Etanercept, Recombinant human TNFR, Fc tagged | 1mg | $1,298.00 |
|
Add to Cart Order |
| 10mg | $3,998.00 |
|
Add to Cart Order | ||
The complement system is a central component of the innate immune system responsible for defending the body against pathogens, clearing apoptotic cells, and modulating inflammation. It functions through a cascade of events initiated by the activation of several complement proteins, ultimately leading to the formation of the membrane attack complex (MAC) and the generation of inflammatory mediators. One of the primary pathways of complement activation is the classical pathway, which is initiated by the binding of the C1 complex to immune complexes. The C1 complex consists of three subunits: C1q, C1r, and C1s. Of these, C1s is a serine protease that plays a critical role in the classical complement pathway, specifically in the activation of C4 and C2, which are necessary for the formation of the C3 convertase enzyme. Because of its essential role in immune responses and inflammation, C1s has become an attractive drug target for various therapeutic applications, particularly in autoimmune diseases, inflammatory disorders and certain infectious diseases.
NCBI Gene ID: 716
UniProtKB ID: P09871
C1s is a serine protease that is an integral component of the C1 complex in the classical complement pathway. The C1 complex is activated by a series of conformational changes upon binding to antigen-antibody complexes. C1s, along with C1r, is part of the C1 complex protease machinery. C1s exists in its inactive form as a zymogen and requires activation to exert its enzymatic function.
The structure of C1s consists of two major domains: an N-terminal serine protease domain and a C-terminal collagen-like domain. The serine protease domain is responsible for the enzymatic activity of C1s, while the collagen-like region facilitates the interaction of C1s with C1q, the recognition component of the C1 complex. Upon activation, C1s cleaves two key complement proteins, C4 and C2, into their active forms. Cleavage of C4 produces C4a and C4b, while cleavage of C2 produces C2a and C2b. The C4b and C2a fragments combine to form the C3 convertase enzyme (C4b2a), which plays a critical role in amplifying the complement cascade.
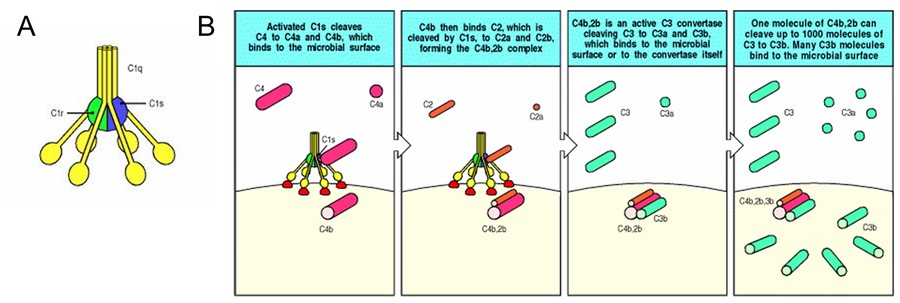 Figure 1. A: C1 system. which is a complex of C1q,
C1r, and C1s. B: The classical pathway of complement activation generates a C3 convertase that deposits large numbers of C3b molecules on the pathogen
surface. (Immunobiology, 5th Edition)
Figure 1. A: C1 system. which is a complex of C1q,
C1r, and C1s. B: The classical pathway of complement activation generates a C3 convertase that deposits large numbers of C3b molecules on the pathogen
surface. (Immunobiology, 5th Edition)
Through this process, C1s helps to propagate the complement cascade, which leads to pathogen opsonization, inflammation, and MAC formation, ultimately resulting in the clearance of pathogens and cellular debris. Regulation of C1s activity is critical because uncontrolled complement activation can lead to tissue damage and the development of inflammatory diseases.
The complement system, with C1s at its core, plays a pivotal role in the immune system's ability to recognize and eliminate pathogens, clear apoptotic cells, and regulate inflammation. As a serine protease in the C1 complex, C1s is central to the initiation of the classical pathway of complement activation triggered by antigen-antibody complexes.
C1s is involved in a wide range of pathological conditions, including autoimmune diseases, inflammatory disorders, and infectious diseases. Dysregulation of C1s activity is a key factor in the pathogenesis of several diseases, particularly those characterized by immune complex deposition and inflammation.
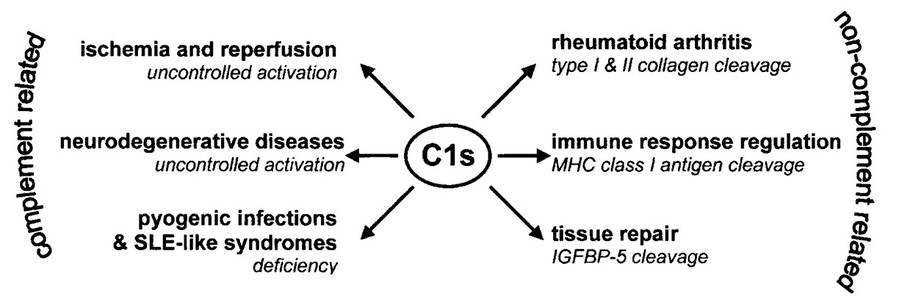 Figure 2. Possible implications of C1s proteolysis
in health and disease. The figure shows the possible physiological processes and diseases in which C1s may participate. Both uncontrolled activation and
deficiency of C1s can lead to severe disorders. The proteolytic action of C1s on non-complement sub strates, although not thoroughly investigated yet, may
also be of importance. (Gal, 2002)
Figure 2. Possible implications of C1s proteolysis
in health and disease. The figure shows the possible physiological processes and diseases in which C1s may participate. Both uncontrolled activation and
deficiency of C1s can lead to severe disorders. The proteolytic action of C1s on non-complement sub strates, although not thoroughly investigated yet, may
also be of importance. (Gal, 2002)
Given its central role in complement activation and its involvement in a variety of diseases, C1s represents a promising therapeutic target. Strategies to modulate C1s activity have the potential to treat a range of conditions, including autoimmune diseases, inflammatory disorders, and certain infectious diseases. Two main approaches to targeting C1s are inhibition and enhancement of its activity.
In autoimmune and inflammatory diseases characterized by excessive complement activation, inhibition of C1s could help reduce tissue damage and inflammation. Several small molecules, monoclonal antibodies and peptide inhibitors have been developed to block C1s activity. These inhibitors can prevent the cleavage of C4 and C2, thereby blocking the formation of C3 convertase and subsequent activation of the complement cascade. By inhibiting C1s, these therapies may limit the inflammatory response and reduce the progression of autoimmune diseases such as SLE and rheumatoid arthritis.
Several complement inhibitors, including those targeting C1s, are currently in clinical development. For example, the monoclonal antibody crovalimab has shown promise in clinical trials as an inhibitor of the classical complement pathway, targeting C1s to prevent complement activation in diseases such as paroxysmal nocturnal hemoglobinuria (PNH) and other complement-mediated disorders.
In infectious diseases, enhancing C1s activity could improve the ability of the immune system to clear pathogens. By promoting activation of the classical complement pathway, C1s enhancement could lead to improved opsonization and pathogen lysis. This approach could be particularly beneficial in the context of infections where the immune system is unable to mount an effective complement response due to complement evasion mechanisms employed by pathogens.
Enhancing C1s activity may also help improve clearance of apoptotic cells in diseases such as cancer, where tumor cells can evade immune surveillance through defective cell death pathways.
Conestat alfa and native human C1-esterase inhibitor indirectly target C1s by inhibiting its protease activity, preventing its role in the complement cascade.
Creative BioMart specializes in providing therapeutic proteins such as conestat alfa and hC1INH that target complement component 1s. Please feel free to contact us with any questions or inquiries.
References
For more information on how our products could help advance your project, please contact us.
ENTER YOUR EMAIL HERE TO SUBSCRIBE.
Copyright © 2026 Creative BioMart. All Rights Reserved.