| Catalog# | Product Name | Size | Price | Qty | Inquiry |
|---|---|---|---|---|---|
| THP-0013 | Conestat alfa; Recombinant C1 esterase inhibitor | 1 vial | $3,998.00 |
|
Add to Cart Order |
| THP-0016 | Native Human C1-esterase inhibitor (hC1INH) | 1 vial | $3,998.00 |
|
Add to Cart Order |
| THP-0112 | Etanercept, Recombinant human TNFR, Fc tagged | 1mg | $1,298.00 |
|
Add to Cart Order |
| 10mg | $3,998.00 |
|
Add to Cart Order | ||
| THP-0152 | Alefacept, Recombinant human LFA3 protein, Fc tagged | 1 vial | $3,998.00 |
|
Add to Cart Order |
The complement system is an integral part of the innate immune system responsible for recognizing and eliminating pathogens, modulating inflammation, and facilitating the clearance of apoptotic cells. It functions through a cascade of reactions that activate a series of proteins, ultimately leading to the formation of the membrane attack complex (MAC) and the opsonization of pathogens. The system is divided into three major pathways: the classical pathway, the alternative pathway and the lectin pathway. The classical complement pathway, which plays a central role in immune defense, is activated when the C1 complex binds to antibody-antigen complexes. Central to this pathway is the C1 complex, which consists of three subunits: C1q, C1r, and C1s. Of these, C1r is a serine protease that plays a critical role in the initiation and regulation of the complement cascade. The study of C1r and its potential as a therapeutic target has attracted increasing interest due to its involvement in a number of pathological conditions, including autoimmune diseases, inflammatory disorders and certain infectious diseases.
NCBI Gene ID: 715
UniProtKB ID: P0036
The C1r protein is an essential component of the C1 complex, which serves as the initiator of the classical complement pathway. Together with C1s, C1r is a serine protease that is activated by conformational changes in the C1q subunit upon binding to antigen-antibody complexes. Structurally, C1r exists as a homodimer composed of two identical subunits, each containing an N-terminal protease domain and a C-terminal collagen-like region. The protease domain is responsible for the enzymatic activity of C1r, while the collagen-like region helps mediate the interactions between C1q and C1r and stabilizes the C1 complex.
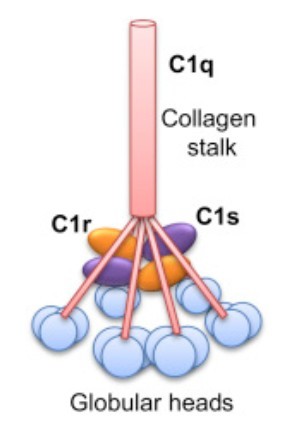 Figure 1. Complement component C1. C1 is comprised
of three different subunits C1q, C1r, and C1s. C1q is produced from six identical subunits which form a collagenous stalk with globular heads at the
carboxy-terminus. The globular heads can recognize and bind to either IgG/IgM or bind directly onto the surface of the target cell. Binding causes a
conformational change which activates C1r to cleave C1s causing it to become enzymatically active. (Middleton et al., 2016)
Figure 1. Complement component C1. C1 is comprised
of three different subunits C1q, C1r, and C1s. C1q is produced from six identical subunits which form a collagenous stalk with globular heads at the
carboxy-terminus. The globular heads can recognize and bind to either IgG/IgM or bind directly onto the surface of the target cell. Binding causes a
conformational change which activates C1r to cleave C1s causing it to become enzymatically active. (Middleton et al., 2016)
In its inactive form, C1r exists as a zymogen that requires activation to become catalytically active. Upon binding of C1q to immune complexes, C1r undergoes a conformational change leading to its activation. Activated C1r then cleaves C1s, another serine protease in the C1 complex, initiating the complement cascade. This cleavage results in the activation of C4 and C2, leading to the formation of the C3 convertase enzyme. C3 convertase then cleaves C3 into C3a and C3b, triggering the downstream effects of the complement system, including opsonization, inflammation, and cell lysis.
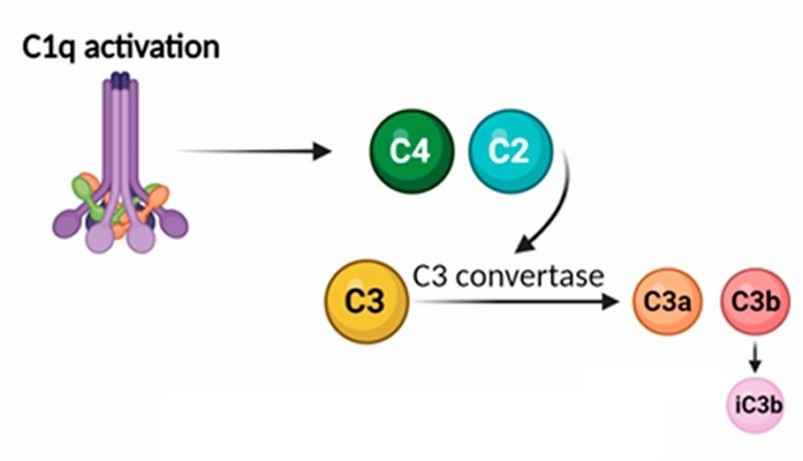 Figure 2. In the classical pathway of the
complement system, C1q binding to an antigen-antibody complex activates C1r, which in turn activates C1s. Activated C1s then cleaves C4 and C2, forming the
C3 convertase (C4b2a) (Adapted from Batista et al., 2024)
Figure 2. In the classical pathway of the
complement system, C1q binding to an antigen-antibody complex activates C1r, which in turn activates C1s. Activated C1s then cleaves C4 and C2, forming the
C3 convertase (C4b2a) (Adapted from Batista et al., 2024)
Thus, C1r is critical for activation of the classical complement pathway and regulation of immune responses. Precise control of C1r activation is essential to maintain immune homeostasis, as excessive or inappropriate complement activation can lead to tissue damage and the development of pathological conditions.
The complement system, including C1r, plays a critical role in the immune system's ability to respond to infection, clear apoptotic cells, and modulate inflammation. In particular, the classical pathway is integral to the body's defense against pathogens and regulation of immune tolerance.
Dysregulation of the complement system, including C1r, has been implicated in a variety of pathological conditions, including autoimmune diseases, inflammatory disorders, and certain infectious diseases. The critical role of C1r in initiating the classical complement pathway makes it a key player in these diseases.
Given its central role in immune responses and disease pathogenesis, C1r represents an attractive target for therapeutic intervention. Several strategies have been proposed to modulate C1r activity, either by inhibition or enhancement, depending on the clinical context.
In diseases characterized by excessive complement activation, such as SLE, rheumatoid arthritis and sepsis, inhibition of C1r may provide therapeutic benefit by preventing overactivation of the complement system. One potential strategy is to develop monoclonal antibodies or small molecules that specifically inhibit the activity of C1r. These therapies could block the activation of C1r, thereby preventing the downstream activation of C1s and the subsequent complement cascade.
Several complement inhibitors are already in clinical use, including eculizumab, a monoclonal antibody that inhibits C5, a downstream complement component. However, targeting earlier components of the complement system, such as C1r, may provide a more precise and effective approach to modulating complement activation.
Conversely, in certain infectious diseases, enhancing C1r activity could improve pathogen clearance by enhancing complement-mediated immune responses. This could be achieved through the development of small molecules or biologics that enhance activation of the classical complement pathway. By increasing C1r activity, these therapies could promote pathogen opsonization, enhance phagocytosis and facilitate MAC formation, leading to more efficient pathogen clearance.
Creative BioMart specializes in providing therapeutic proteins, including conestat alfa and hC1INH, targeting complement component 1r. Contact us with any questions or inquiries.
References
For more information on how our products could help advance your project, please contact us.
ENTER YOUR EMAIL HERE TO SUBSCRIBE.
Copyright © 2026 Creative BioMart. All Rights Reserved.