| Catalog# | Product Name | Size | Price | Qty | Inquiry |
|---|---|---|---|---|---|
| THP-0112 | Etanercept, Recombinant human TNFR, Fc tagged | 1mg | $1,298.00 |
|
Add to Cart Order |
| 10mg | $3,998.00 |
|
Add to Cart Order | ||
| THP-0152 | Alefacept, Recombinant human LFA3 protein, Fc tagged | 1 vial | $3,998.00 |
|
Add to Cart Order |
The complement system is a crucial component of the innate immune response, composed of over 30 proteins that act in concert to defend the body against infections, promote inflammation, and clear apoptotic cells. It is divided into three major pathways: the classical pathway, the alternative pathway, and the lectin pathway, all of which converge on a common terminal pathway leading to the formation of the membrane attack complex (MAC). Central to the classical pathway is the C1 complex, which consists of three subunits: C1q, C1r and C1s. Of these, C1q is a key molecule because it initiates the classical pathway by binding to antibodies or pathogen-associated molecular patterns (PAMPs). C1q is composed of three distinct chains: C1qA, C1qB and C1qC. Each chain plays a critical role in the functionality of C1q, and their molecular interactions are essential for the initiation of immune responses. The C1QB gene encodes the B chain of C1q, and its unique structural and functional properties position it as a promising target for drug development, particularly in the context of immune modulation and autoimmune diseases.
NCBI Gene ID: 713
UniProtKB ID: P02746
The C1q complex is a crucial initiator of the classical complement pathway, and its structure has been extensively studied. C1q is composed of six subunits, arranged in three heterotrimers, each containing one A, one B, and one C chain. These chains are characterized by a long, collagen-like region and a globular head domain. The C1qB chain, in particular, is integral to the function of C1q, as it contributes to the recognition of antigen-antibody complexes, which is a key event in the initiation of the classical complement cascade.
The C1qB chain consists of approximately 200 amino acids and is characterized by an extended triple helix in the collagen-like region and a globular head at the C-terminal end. The globular head contains the binding sites for C1r and C1s, the serine proteases that, upon activation, cleave and activate subsequent complement proteins. This structural organization is critical for target recognition and subsequent activation of the complement cascade.
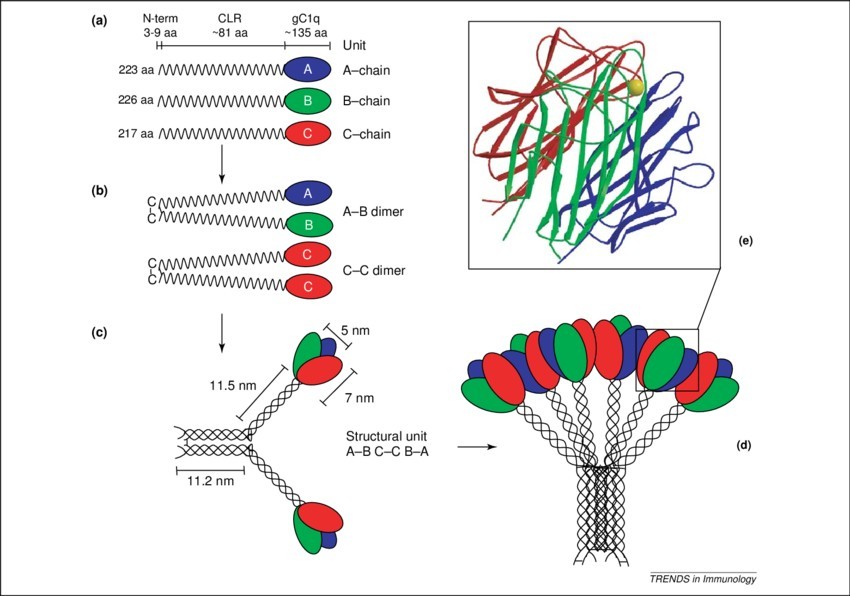 Figure 1. Structural organization of the C1q
molecule. C1q (460 kDa) is composed of 18 polypeptide chains. (a) The A, B and C chains each have a short N-terminal region, followed by a collagen region
(CLR) of w81 residues and a C-terminal globular region (gC1q domain) of w135 residues. (b) The interchain disulfide bonding yields 6A–B dimer subunits and
3C-C dimer subunits. The triple-helical collagen region in the A and B chains of an A–B subunit, together with one of the C-chains present in a C–C subunit,
form a structural unit (ABC–CBA), which is held together by both covalent and non-covalent bonds (c). Three of these structural units associate, via strong
non-covalent bonds in the fibril-like central portion, to yield the hexameric C1q molecule that has a tulip-like structure (d). The crystal structure of the
gC1q domain of human C1q (Protein Data Bank code 1PK6, depicted as a ribbon diagram of ghA in blue, ghB in green, ghC in red, with the calcium ion shown as
a yellow ball), has revealed a compact, spherical, heterotrimeric assembly (50 A diameter), held together predominantly by non-polar interactions, with
non-crystallographic pseudo-threefold symmetry (e). The three gC1q modules show clear differences in their electrostatic surface potentials, which in part
explains modularity in terms of ligand recognition. (Kishore et al., 2004)
Figure 1. Structural organization of the C1q
molecule. C1q (460 kDa) is composed of 18 polypeptide chains. (a) The A, B and C chains each have a short N-terminal region, followed by a collagen region
(CLR) of w81 residues and a C-terminal globular region (gC1q domain) of w135 residues. (b) The interchain disulfide bonding yields 6A–B dimer subunits and
3C-C dimer subunits. The triple-helical collagen region in the A and B chains of an A–B subunit, together with one of the C-chains present in a C–C subunit,
form a structural unit (ABC–CBA), which is held together by both covalent and non-covalent bonds (c). Three of these structural units associate, via strong
non-covalent bonds in the fibril-like central portion, to yield the hexameric C1q molecule that has a tulip-like structure (d). The crystal structure of the
gC1q domain of human C1q (Protein Data Bank code 1PK6, depicted as a ribbon diagram of ghA in blue, ghB in green, ghC in red, with the calcium ion shown as
a yellow ball), has revealed a compact, spherical, heterotrimeric assembly (50 A diameter), held together predominantly by non-polar interactions, with
non-crystallographic pseudo-threefold symmetry (e). The three gC1q modules show clear differences in their electrostatic surface potentials, which in part
explains modularity in terms of ligand recognition. (Kishore et al., 2004)
Functionally, the primary role of C1qB is to facilitate recognition of immune complexes formed when antibodies bind to antigens on the surface of pathogens or damaged cells. Upon binding, C1q activates C1r and C1s, leading to the cleavage of C4 and C2 proteins. This process ultimately leads to the formation of the enzyme C3 convertase, which cleaves C3 into C3a and C3b. C3b is a central component of the complement system, mediating opsonization of pathogens and formation of MAC, which induces lysis of the target cell. Thus, the function of C1qB, through its role in activating the classical pathway, is essential for both innate immune defense and immune regulation.
C1q, and by extension C1qB, is critical for the immune response to a wide range of pathogens, including bacteria, viruses and fungi. It also plays an important role in clearing apoptotic cells and maintaining immune tolerance. However, dysregulation of C1q function has been implicated in a variety of autoimmune and inflammatory diseases.
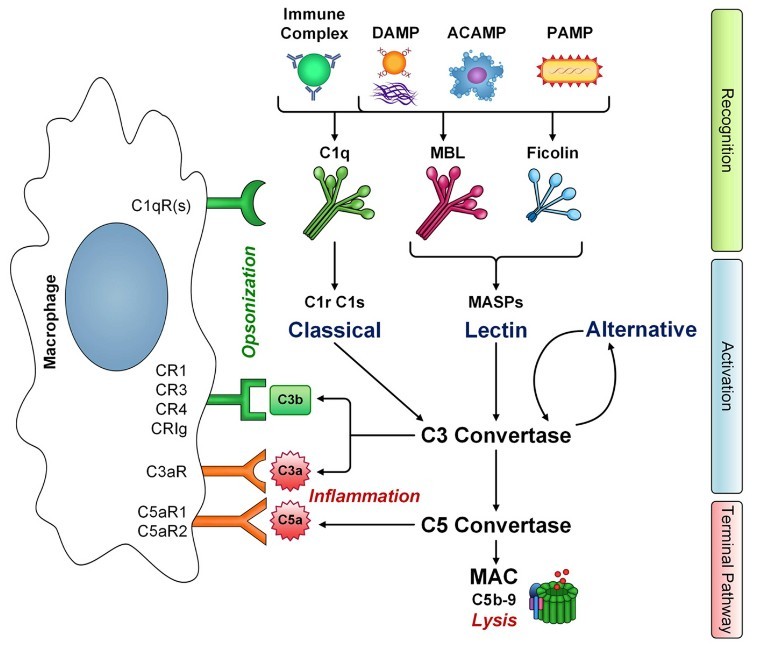 Figure 2. The interaction of macrophages with
complement. Innate immune pattern recognition receptors C1q, MBL, and ficolins recognize a number of structures including immune complexes, damaged-self
molecules expressing damage associated molecular patterns (DAMPS), apoptotic cells (ACAMPs), and pathogens (PAMPs), leading to activation of classical and
lectin pathways, respectively, and amplification via the alternative pathway. (Bohlson et al., 2014)
Figure 2. The interaction of macrophages with
complement. Innate immune pattern recognition receptors C1q, MBL, and ficolins recognize a number of structures including immune complexes, damaged-self
molecules expressing damage associated molecular patterns (DAMPS), apoptotic cells (ACAMPs), and pathogens (PAMPs), leading to activation of classical and
lectin pathways, respectively, and amplification via the alternative pathway. (Bohlson et al., 2014)
The involvement of C1q and its components in disease pathogenesis has been well-documented in various autoimmune, inflammatory, and infectious diseases. The C1qB chain, as part of the C1q complex, plays a critical role in these processes.
Given its central role in the immune response and disease pathogenesis, C1q, particularly C1qB, represents a promising therapeutic target. Targeting C1q offers several potential strategies to modulate the complement system in a range of diseases, from autoimmune to infectious diseases.
Are you interested in therapeutic proteins? Please don't hesitate to contact us!
References
For more information on how our products could help advance your project, please contact us.
ENTER YOUR EMAIL HERE TO SUBSCRIBE.
Copyright © 2026 Creative BioMart. All Rights Reserved.