Cat# : THA-0092
| Cat#: | THA-0092 |
| Product Name: | Moxetumomab pasudotox |
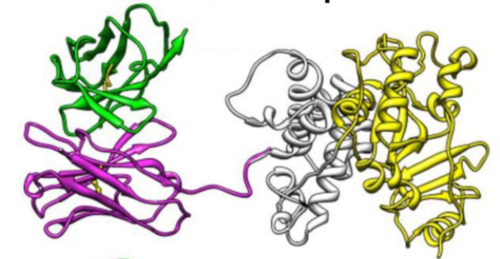
| Description: | Moxetumomab pasudotox is a CD22-specific antibody conjugated to a truncated exotoxin used to treat relapsed or refractory hairy cell leukemia in patients who have already been treated with a purine nucleoside analog and one other treatment. |
| Sequences: | VH-P38 subunit: MEVQLVESGGGLVKPGGSLKLSCAASGFAFSIYDMSWVRQTPEKCLEWVAYISSGGGTTYYPDTVKGRFTISRDNAKNTLYLQMSSLKSEDTAMYYCARHSGYGTHWGVLFAYWGQGTLVSAKASGGPEGGSLAALTAHQACHLPLETFTRHRQPRGWEQLEQCGYPVQRLVALYIAARLSWNQVDQVIRALASPGSGGDLGEAIREQPEQARLALTLAAAESERFVRQGTGNDEAGAANGPADSGDALLERNYPTGAEFLGDGGDVSFSTRGTQNWTVERLLQAHRQLEERGYVFVGYHGTFLEAAQSIVFGGVRARSQDLDAIWRGFYIAGDPALAYGYAQDQEPDARGRIRNGALLRVYVPRSSLPGFYRTSLTLAAPEAAGEVERLIGHPLPLRLDAITGPEEEGGRLETILGWPLAERTWIPSAIPTDPRNVGGDLDPSSIPDKEQAISALPDYASQPGKPPREDLK VL subunit: MDIQMTQTTSSLSASLGDRVTISCRASQDISNYLNWYQQKPDGTVKLLIYYTSILHSGVPSRFSGSGSGTDYSLTISNLEQEDFATYFCQQGNTLPWTFGCGTKLEIK PE-38 of Moxetumomab pasudotox: PEGGSLAALTAHQACHLPLETFTRHRQPRGWEQLEQCGYPVQRLVALYLAARLSWNQVDQVIRNALASPGSGGDLGEAIREQPEQARLALTLAAAESERFVRQGTGNDEAGAANGPADSGDALLERNYPTGAEFLGDGGDVSFSTRGTQNWTVERLLQAHRQLEERGYVFVGYHGTFLEAAQSIVFGGVRARSQDLDAIWRGFYIAGDPALAYGYAQDQEPDARGRIRNGALLRVYVPRSSLPGFYRTSLTLAAPEAAGEVERLIGHPLPLRLDAITGPEEEGGRLETILGWPLAERTVVIPSAIPTDPRNVGGDLDPSSIPDKEQAISALPDYASQPGKPPREDLK |
| Molecular Weight: | 63500.0 Da |
| Introduction: | CD22 is a lineage-restricted B-cell antigen that is expressed solely in on B-chronic lymphocytic leukemia, hairy cell leukemia, acute lymphocytic leukemiathe and Burkitt's lymphoma. The predecessor of Moxetumab pasudotox (MxP), named BL22, was first created based on the antibody RFB4 which specifically binds to CD22. This antibody was used to generate a recombinant immunotoxin in which a stabilized Fv segment by a disulfide bond is fused to the Pseudomonas exotoxin A (PE38) which does not have the cell-binding portion. MxP appears as an improved form of BL22 by the mutation of the Fv region and the antibody phage-displayed. As well the residues SSY in the heavy chain are mutated to THW. It was developed by Astra Zeneca and FDA approved on September 13, 2018, after being granted the status of Fast Track, Priority Review and Orphan Drug designations. |
| Purity: | >99% by SDS-Page and HPLC analysis |
| Formula: | C2804H4339N783O870S14 |
| Appearance: | Solid |
| Endotoxin Level: | < 0.001 EU/μg of the protein by the LAL method |
|
|
For more information on how our products could help advance your project, please contact us.
ENTER YOUR EMAIL HERE TO SUBSCRIBE.
Copyright © 2026 Creative BioMart. All Rights Reserved.