| Catalog# | Product Name | Size | Price | Qty | Inquiry |
|---|---|---|---|---|---|
| THP-0020 | Tenecteplase; Modified Tissue plasminogen activator (tPA) | 100ug | $798.00 |
|
Add to Cart Order |
| 500ug | $2,498.00 |
|
Add to Cart Order | ||
| 1mg | $3,998.00 |
|
Add to Cart Order | ||
Annexin A2 (encoded by the gene ANXA2) is a calcium-dependent phospholipid-binding protein involved in cellular processes such as membrane repair, exocytosis, angiogenesis, and fibrinolysis. Dysregulation of annexin A2 expression and function has been implicated in various pathological conditions, including cancer metastasis, thrombotic disorders, and inflammatory diseases. As a surface receptor for tissue plasminogen activator (tPA) and plasminogen, annexin A2 plays a critical role in fibrinolysis, making it an attractive therapeutic target.
NCBI Gene ID: 302
UniProtKB ID: P07355
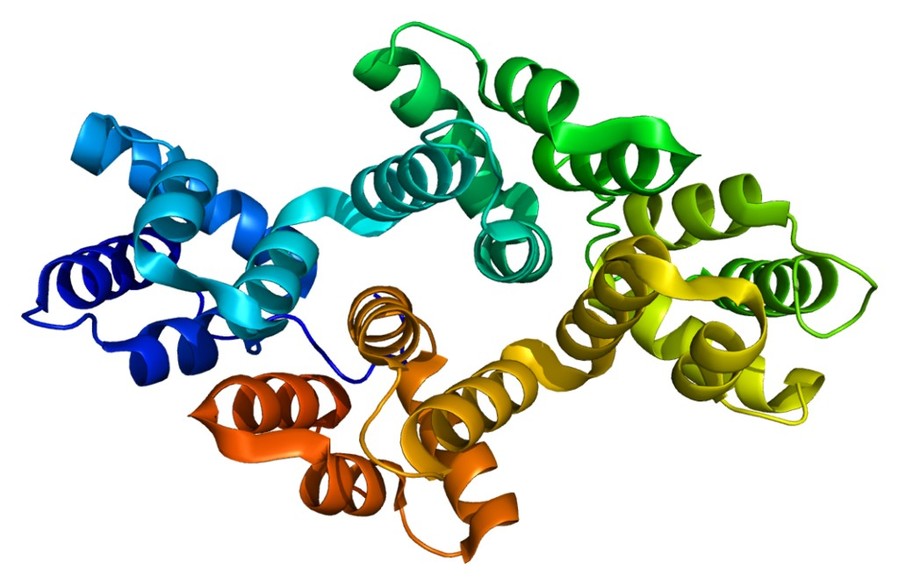 Figure 1. Structure of the ANXA2 protein, PDB
code: 1W7B.
Figure 1. Structure of the ANXA2 protein, PDB
code: 1W7B.
Annexin A2 has a structure consisting of a highly conserved carboxyl-terminal core domain with four repeating segments of about 70 amino acids each, forming a curved shape with a convex and concave side, and a variable amino-terminal "head" domain that is the site for protein-protein interactions and post-translational modifications; the core domain contains binding sites for calcium ions and anionic phospholipids, while the N-terminal domain interacts with other proteins, such as S100A10, through a hydrophobic patch; each repeat in the core domain is composed of five alpha helices (A-E), four of which are antiparallel and the fifth is perpendicular, forming a right-handed superhelix.
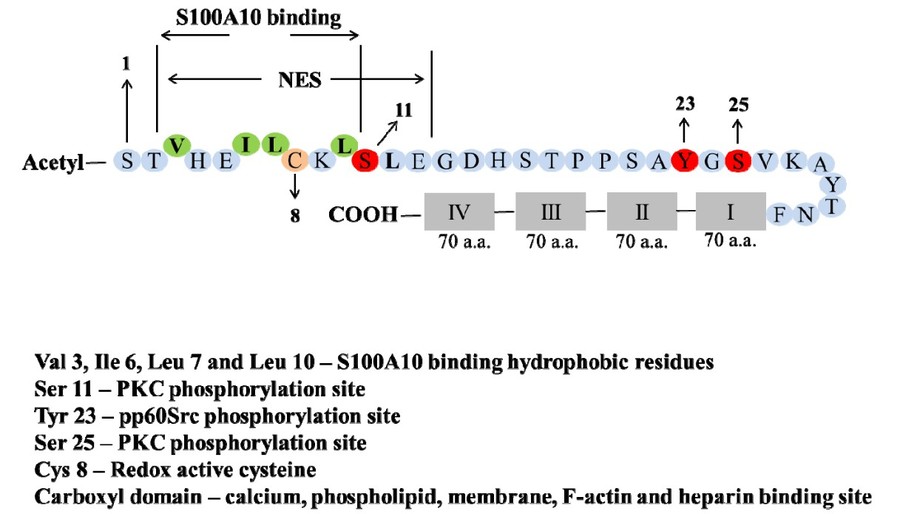 Figure 2. Domain structure of annexin A2.
Annexin A2 is composed to two domains—the amino-terminal domain and carboxyl-terminal domain. The amino-terminal is the site for post-translational
modifications (Ser-1–Phe-32) such as acetylation (Ser-1) and phosphorylation (Ser-11, Tyr-23, Ser-25). Additionally, it also encompasses the redox reactive
cysteine residue (Cys-8) and the nuclear export sequence (NES) (Val-3-Leu-12). The S100A10 binding site is an amphipathic α-helix, with the hydrophobic
residues, Val-3, Ile-6, Leu-7 and Leu-10 making contacts with S100A10. The carboxyl-terminal core domain includes four predominantly alpha-helical domains
each containing 70 amino acids. This carboxyl-terminal core domain contains binding sites for heparin and RNA, calcium and phospholipid and as well as for
F-actin. (Bharadwaj et al., 2013)
Figure 2. Domain structure of annexin A2.
Annexin A2 is composed to two domains—the amino-terminal domain and carboxyl-terminal domain. The amino-terminal is the site for post-translational
modifications (Ser-1–Phe-32) such as acetylation (Ser-1) and phosphorylation (Ser-11, Tyr-23, Ser-25). Additionally, it also encompasses the redox reactive
cysteine residue (Cys-8) and the nuclear export sequence (NES) (Val-3-Leu-12). The S100A10 binding site is an amphipathic α-helix, with the hydrophobic
residues, Val-3, Ile-6, Leu-7 and Leu-10 making contacts with S100A10. The carboxyl-terminal core domain includes four predominantly alpha-helical domains
each containing 70 amino acids. This carboxyl-terminal core domain contains binding sites for heparin and RNA, calcium and phospholipid and as well as for
F-actin. (Bharadwaj et al., 2013)
Annexin A2 serves as a coreceptor for tissue plasminogen activator (tPA) and plasminogen on endothelial cell surfaces, facilitating the conversion of plasminogen to plasmin. Plasmin is a critical enzyme responsible for degrading fibrin, thereby preventing excessive fibrin accumulation and maintaining vascular patency. This fibrinolytic activity underscores the essential role of annexin A2 in regulating clot lysis and ensuring proper hemostasis.
In addition to its fibrinolytic functions, annexin A2 significantly influences inflammatory responses. It modulates the behavior of immune cells, including macrophages and monocytes, affecting cytokine production and secretion. This modulation can either enhance or attenuate inflammatory processes, depending on the context. Dysregulation of annexin A2 has been associated with several autoimmune diseases, suggesting that maintaining its proper function is critical for the balance of the immune system.
Annexin A2 also contributes to tissue repair mechanisms. It influences cellular processes such as proliferation, migration and adhesion, which are essential for effective wound healing. However, aberrant annexin A2 activity can lead to pathological fibrosis characterized by excessive extracellular matrix deposition and tissue scarring. This highlights the dual role of annexin A2 in both promoting normal tissue repair and contributing to fibrotic diseases when its regulation is disrupted.
Given its involvement in fibrinolysis, angiogenesis, and inflammation, annexin A2 is a promising target for therapeutic interventions. Several strategies have been developed to modulate its activity:
Tenecteplase (Modified tPA): Tenecteplase is a genetically engineered variant of tissue plasminogen activator with enhanced fibrinolytic activity and improved pharmacokinetic properties. By binding to annexin A2, tenecteplase enhances plasmin generation and effectively dissolves blood clots. It is widely used in the treatment of acute myocardial infarction.
Key Modifications and Advantages
Tenecteplase differs from native tPA in three key amino acid substitutions that improve its therapeutic efficiency:
Clinical Applications
Therapeutic antibodies targeting annexin A2 aim to block its interaction with plasminogen and tPA, reducing plasmin generation. These approaches have shown potential in preclinical studies for thrombotic and metastatic diseases.
While annexin A2 holds great promise as a drug target, several challenges remain:
Annexin A2 is a versatile protein that plays a central role in fibrinolysis, angiogenesis and inflammation. Its dysregulation in pathological conditions makes it an attractive target for therapeutic intervention. Recombinant proteins such as tenecteplase have already demonstrated the efficacy of targeting annexin A2 in thrombotic diseases, while newer therapies, including monoclonal antibodies and RNA-based approaches, show promise in cancer and inflammatory diseases.
Creative BioMart provides high-quality recombinant proteins including tenecteplase, contact us for inquiry and any questions.
References
For more information on how our products could help advance your project, please contact us.
ENTER YOUR EMAIL HERE TO SUBSCRIBE.
Copyright © 2026 Creative BioMart. All Rights Reserved.