| Catalog# | Product Name | Size | Price | Qty | Inquiry |
|---|---|---|---|---|---|
| THP-0100 | Von Willebrand Factor(VWF) Human | 625 IU | $3,998.00 |
|
Add to Cart Order |
ADAMTS13, a thrombospondin type 1 motif disintegrin and metalloproteinase—also known as von Willebrand factor-cleaving protease (VWFCP)—is a key enzyme in hemostasis. It cleaves ultra-large von Willebrand factor (UL-VWF) multimers to regulate platelet adhesion and aggregation. Dysregulation of ADAMTS13 activity is implicated in thrombotic thrombocytopenic purpura (TTP) and other thrombotic microangiopathies (TMAs). Targeting ADAMTS13, either to increase its activity in deficiency states or to inhibit its function in specific pathological conditions, has therapeutic potential with applications ranging from the treatment of TTP to the mitigation of thrombotic complications in inflammatory or cardiovascular diseases.
NCBI Gene ID: 11093
UniProtKB ID: Q76LX8
ADAMTS13 specifically cleaves the VWF A2 domain at the Tyr1605-Met1606 bond, thereby reducing the adhesive capacity of UL-VWF multimers. Under physiological conditions, this activity prevents excessive platelet adhesion to the vascular endothelium, particularly in high-shear environments such as arterioles. Deficiency or inhibition of ADAMTS13 results in the accumulation of UL-VWF, leading to microvascular thrombosis and organ damage. Conversely, excessive ADAMTS13 activity can lead to reduced hemostatic function, contributing to bleeding disorders.
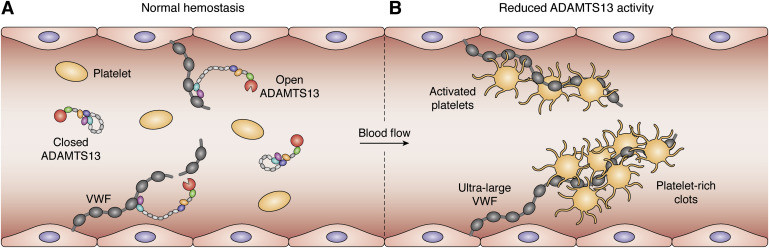 Figure 1. Schematic representation of ADAMTS13 activity. A, normal hemostasis: under physiological conditions, multimeric size of the VWF is controlled by ADAMTS13, which circulates in closed conformation and only adopts a transient open conformation upon interacting with its substrate VWF. B, reduced ADAMTS13 activity leads to accumulation of ULVWF multimers, which promote formation of platelet-rich clots in the microvasculature, giving rise to thrombotic thrombocytopenic purpura. ADAMTS13, a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13; VWF, von Willebrand factor; ULVWF, ultra large von Willebrand factor. (Ercig et al., 2021)
Figure 1. Schematic representation of ADAMTS13 activity. A, normal hemostasis: under physiological conditions, multimeric size of the VWF is controlled by ADAMTS13, which circulates in closed conformation and only adopts a transient open conformation upon interacting with its substrate VWF. B, reduced ADAMTS13 activity leads to accumulation of ULVWF multimers, which promote formation of platelet-rich clots in the microvasculature, giving rise to thrombotic thrombocytopenic purpura. ADAMTS13, a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13; VWF, von Willebrand factor; ULVWF, ultra large von Willebrand factor. (Ercig et al., 2021)
Thrombotic thrombocytopenic purpura is a life-threatening disorder characterized by ADAMTS13 deficiency due to genetic mutations (congenital TTP) or autoantibodies (acquired TTP). The resulting UL-VWF accumulation leads to platelet aggregation, microvascular thrombosis, and organ ischemia. Increasing ADAMTS13 activity is the cornerstone of TTP treatment. Therapeutic approaches include:
While much of the focus has been on enhancing ADAMTS13 activity, emerging evidence suggests that inhibiting ADAMTS13 may be beneficial in certain bleeding disorders where reduced VWF activity exacerbates bleeding risk. In this context, small molecule inhibitors or monoclonal antibodies against ADAMTS13 are being investigated.
ADAMTS13 activity has been implicated in several cardiovascular and inflammatory diseases, including myocardial infarction, stroke and sepsis-associated coagulopathy. Modulation of ADAMTS13 activity may reduce thrombotic complications in these diseases. Therapies targeting ADAMTS13 to fine-tune its activity may minimize pathological thrombosis while preserving normal hemostasis.
Von Willebrand Factor (VWF) Human is a therapeutic drug primarily indicated for the treatment of von Willebrand disease (VWD), a bleeding disorder caused by a deficiency or dysfunction of VWF, which plays a critical role in blood clotting. This drug is derived from human plasma or produced recombinantly to supplement deficient or defective VWF in patients.
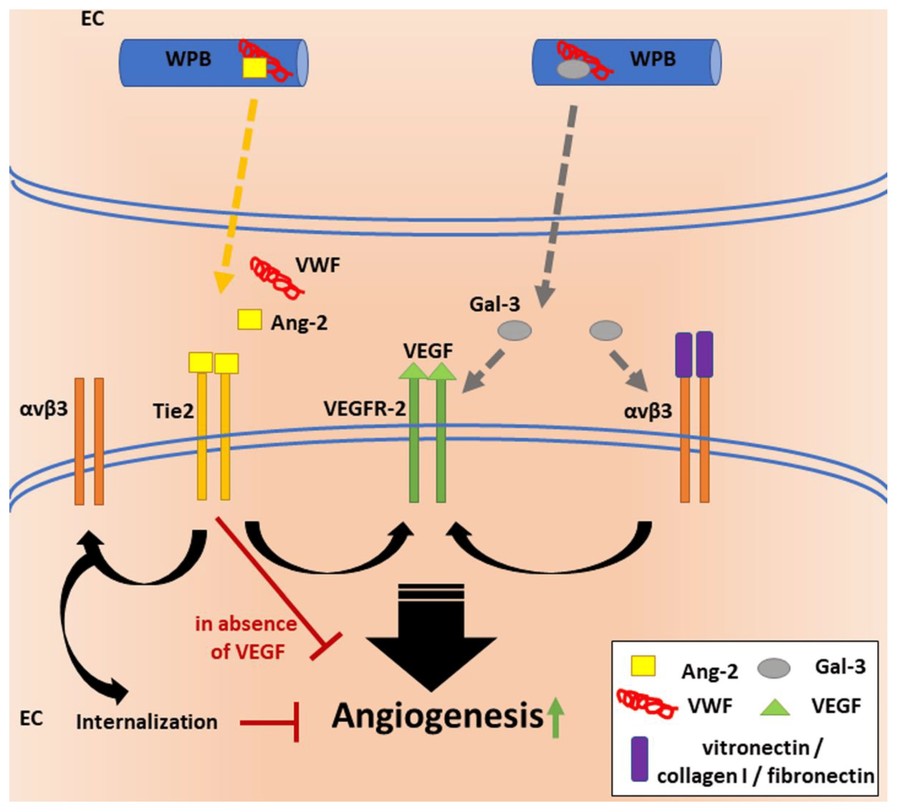 Figure 2. VWF's regulatory functions in angiogenesis. VWF is essential for the formation of WPBs in endothelial cells (ECs) and regulates the storage and release of cargo proteins, such as growth factor Ang-2. Release of Ang-2 and subsequent binding to its receptor Tie-2 can destabilize blood vessels and promote angiogenesis cooperatively with VEGF signaling through VEGFR-2. In contrast, Tie-2 signaling has anti-angiogenic effects in the absence of VEGF. Ligand binding (e.g., vitronectin, fibronectin, collagen I) to αvβ3 has been shown to increase VEGFR-2 activity, to initiate downstream signaling and to thereby increase EC proliferation. Another protein stored in WPBs is the glycan-binding protein Gal-3. Gal-3 promotes angiogenesis through pathways that involve both αvβ3 and VEGFR-2. Thus, VWF may regulate angiogenesis at multiple levels. Intracellularly, it has anti-angiogenic effects when regulating the storage of proangiogenic factors, but it might also function as a proangiogenic extracellular ligand. (Mojzisch and Brehm, 2021)
Figure 2. VWF's regulatory functions in angiogenesis. VWF is essential for the formation of WPBs in endothelial cells (ECs) and regulates the storage and release of cargo proteins, such as growth factor Ang-2. Release of Ang-2 and subsequent binding to its receptor Tie-2 can destabilize blood vessels and promote angiogenesis cooperatively with VEGF signaling through VEGFR-2. In contrast, Tie-2 signaling has anti-angiogenic effects in the absence of VEGF. Ligand binding (e.g., vitronectin, fibronectin, collagen I) to αvβ3 has been shown to increase VEGFR-2 activity, to initiate downstream signaling and to thereby increase EC proliferation. Another protein stored in WPBs is the glycan-binding protein Gal-3. Gal-3 promotes angiogenesis through pathways that involve both αvβ3 and VEGFR-2. Thus, VWF may regulate angiogenesis at multiple levels. Intracellularly, it has anti-angiogenic effects when regulating the storage of proangiogenic factors, but it might also function as a proangiogenic extracellular ligand. (Mojzisch and Brehm, 2021)
VWF facilitates platelet adhesion and aggregation at sites of vascular injury, stabilizing Factor VIII and preventing excessive bleeding. However, its function is regulated by ADAMTS13, a protease that cleaves ultra-large VWF multimers to prevent excessive clot formation.
In cases of ADAMTS13 deficiency, such as thrombotic thrombocytopenic purpura (TTP), unregulated ultra-large VWF multimers lead to microvascular thrombosis. While human VWF is not typically used to directly target ADAMTS13, it plays a role in balancing VWF levels when ADAMTS13 activity is impaired. In contrast, recombinant ADAMTS13 or plasma exchange therapy is used to treat TTP.
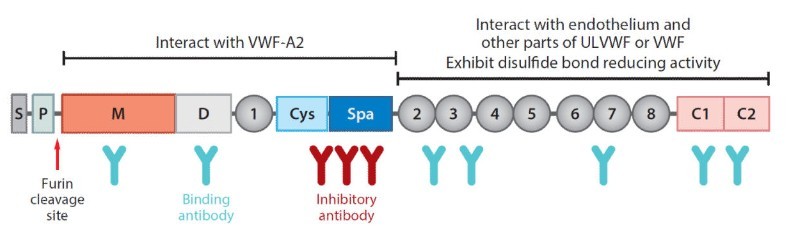 Figure 3. Schematic representation of ADAMTS13 domain organization, potential role in substrate recognition, and binding sites of autoantibodies in acquired TTP. S, signal peptide; P, propeptide; M, metalloprotease domain; D, disintegrin domain; 1, the first thrombospondin type 1 (TSP1) repeat; Cys, the cysteine-rich domain; Spa, the spacer domain; 2–8, TSP1 2–8 repeats; C1 and C2, the first and second CUB domain. Other abbreviations: ULVWF, ultralarge von Willebrand factor; VWF, von Willebrand factor; A2, the A2 domain of VWF. (Zheng, 2015)
Figure 3. Schematic representation of ADAMTS13 domain organization, potential role in substrate recognition, and binding sites of autoantibodies in acquired TTP. S, signal peptide; P, propeptide; M, metalloprotease domain; D, disintegrin domain; 1, the first thrombospondin type 1 (TSP1) repeat; Cys, the cysteine-rich domain; Spa, the spacer domain; 2–8, TSP1 2–8 repeats; C1 and C2, the first and second CUB domain. Other abbreviations: ULVWF, ultralarge von Willebrand factor; VWF, von Willebrand factor; A2, the A2 domain of VWF. (Zheng, 2015)
Future research should focus on the development of highly specific modulators of ADAMTS13 activity. Gene therapy approaches, such as delivery of ADAMTS13 using adeno-associated viral (AAV) vectors, hold promise for the long-term treatment of congenital TTP. In addition, high-throughput screening for small molecule inhibitors or activators may expand the therapeutic armamentarium for ADAMTS13-targeted therapies. Advances in structural biology may also facilitate the design of more refined therapeutic proteins and antibodies.
Creative BioMart is a leading supplier of therapeutic proteins, including Human Von Willebrand Factor (VWF), targeting ADAMTS13. Contact us for more information!
References
For more information on how our products could help advance your project, please contact us.
ENTER YOUR EMAIL HERE TO SUBSCRIBE.
Copyright © 2026 Creative BioMart. All Rights Reserved.